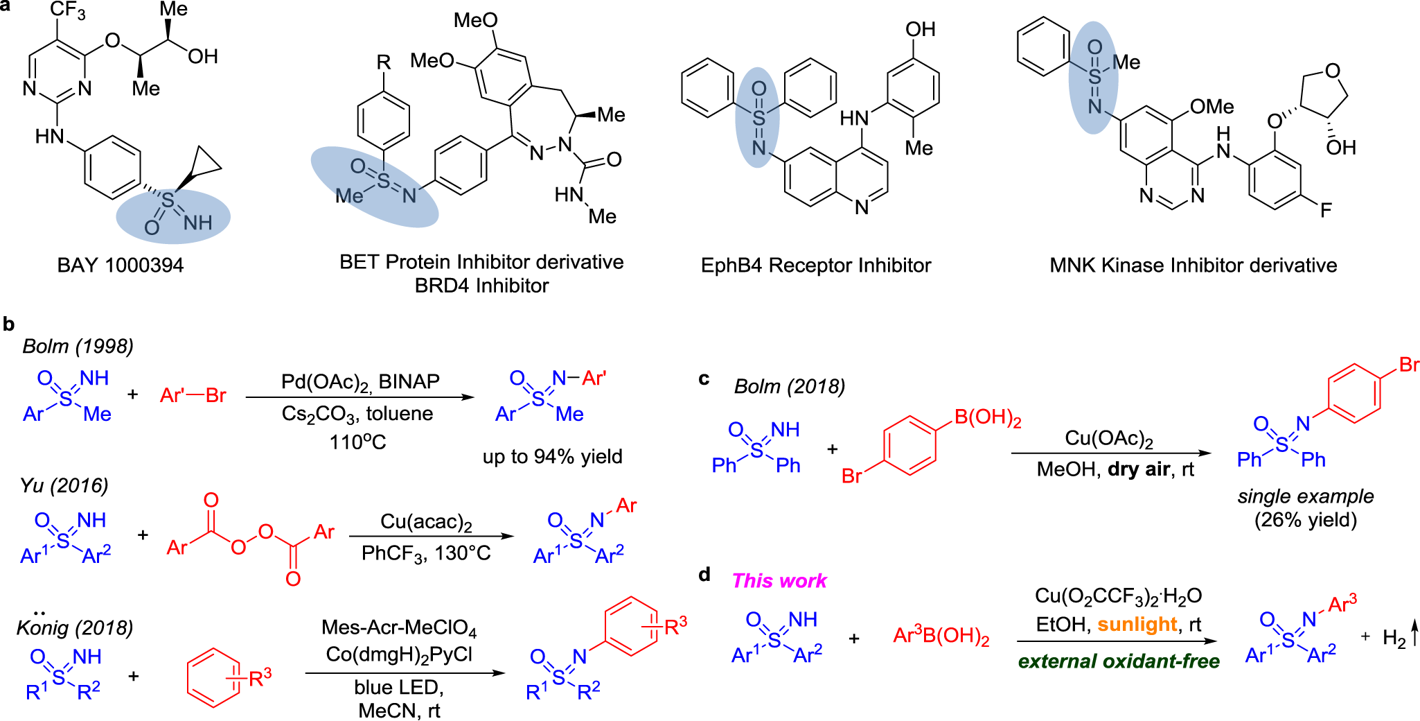
We have commented in previous entries of this blog that the metal-mediated formation of C–N bonds has evolved nicely since Ullmann’s times. Among the options available is the Chan–Lam reaction, which unfortunately remains underdeveloped. It has been available now for 20 years but, although initially quite attractive, it has not seen as much use as Buchwald–Hartwig transformations.
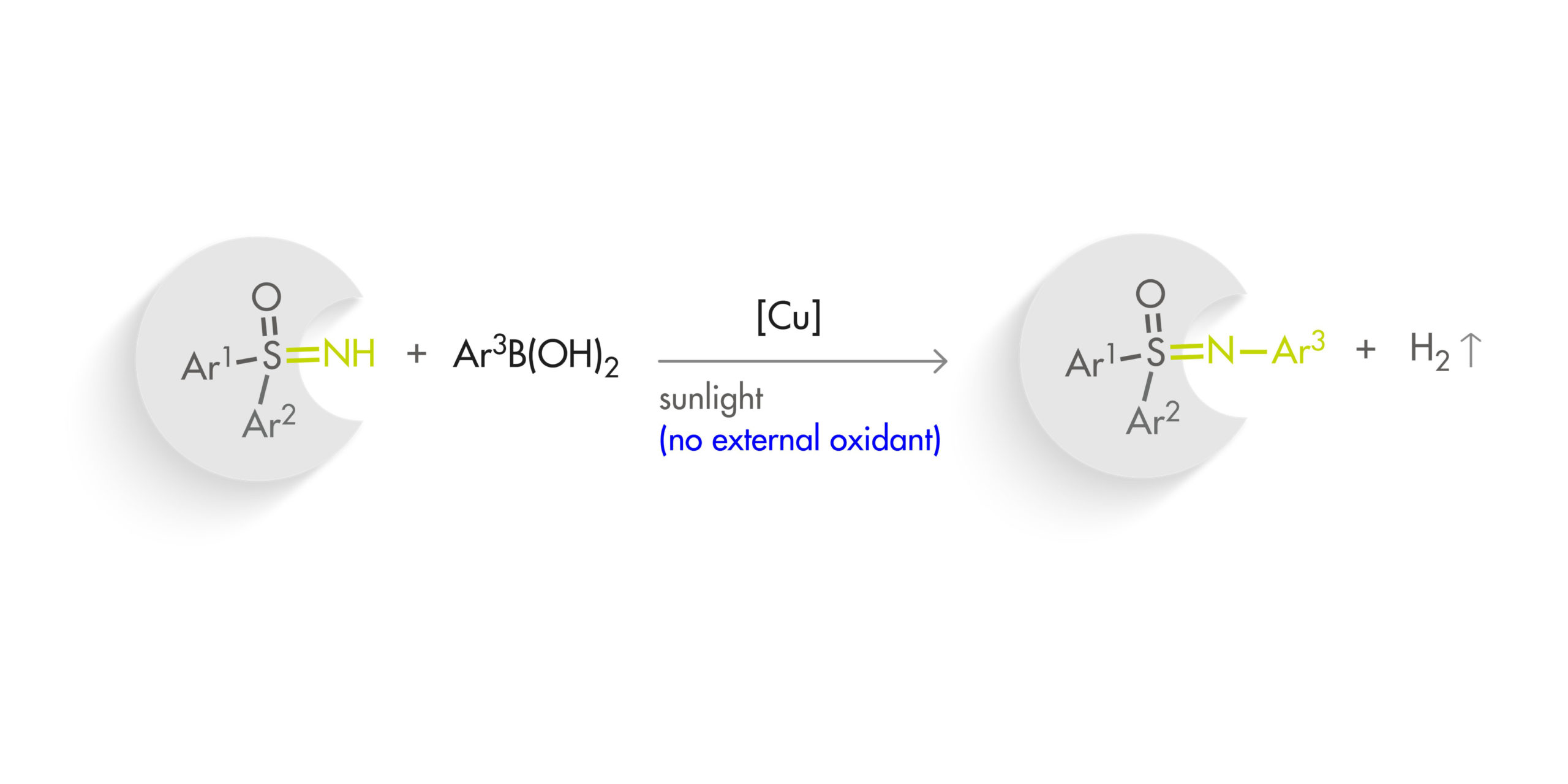
One of the most annoying issues is the lack of proper understanding of the mechanism. The Chan–Lam reaction is copper-based, with amines and (hetero)arylboronic acids as coupling partners, so basically it involves the coupling of two nucleophiles. This means that the reaction needs an oxidant, with oxygen from the atmosphere usually taking this role. The oxidant is sometimes troublesome, and it is very difficult to enhance the performance of a reaction that works in mysterious ways. Along the years, many authors have proposed a number of mechanisms to explain the observed reactivity of such a system with different oxidants, substrates and so. The development of an oxidant-free Chan–Lam reaction would be thus highly attractive.
This paper by Kozlowski and Jia addresses the problem using a modern approach: a copper-catalyzed photoredox dehydrogenative Chan–Lam reaction. The protocol has been developed for the N-arylation of sulfoximines, an emerging motif in medicinal chemistry. In a typical reaction, a diphenylsulfoximine is coupled with a phenylboronic acid in the presence of Cu(CF3CO2)2 (10 mol%) under ambient light in EtOH at r.t. for 48 h. Just like that. The scope of the reaction includes a lot of para-monosubstituted phenylboronic acids (although evaluation of the reaction with meta and specially ortho substrates would have been desirable as well) and even some heterocycles. The aryl rings on the sulfoximine partner can also bear substituents and the reaction seems robust.
A rather striking point of the new method is that the reaction does not need a photocatalyst: the copper (I) complex formed in situ from sulfoximine and the arylation product seems to be responsible for the autocatalysis. The authors carried out several mechanistic experiments, including absence of light, another oxidant, and a radical trapping agent. Indeed, the reaction autocatalyzes itself in the presence of light.
Autocatalytic photoredox Chan–Lam coupling of free diaryl sulfoximines with arylboronic acids. Nat. Commun. 2021,12, 932. See:10.1038/s41467-021-21156-w