Engaging Nonaromatic, Heterocyclic Tosylates in Reductive Cross-Coupling with Aryl and Heteroaryl Bromides
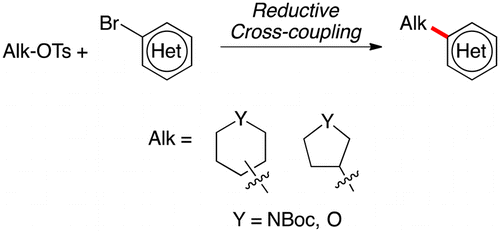
Reductive coupling of common aliphatic heterocycles (piperidines, pyrrolidines, pyranes and so) with aryl bromides
When you are running med chem projects in a continuous basis, you are used to consider how to introduce some common motifs. For example, how to attach a 4-piperidyl ring into an heterocycle. This is not an impossible transformation and there are several options available. But some of these options will leave you with intermediates that you have to process further (i.e., alkenes that you have to reduce, etc.). So new simple methods to carry out these transformations are always welcomed; new additions to the ever growing weaponry in organic synthesis.
Molander et al. (Pennsylvania University, PA, USA) have published such a contribution, focused in the introduction of nonaromatic, heterocyclic tosylates into aromatic and heteroaromatic rings. In summary, they have improved previous conditions making use of an apparently cumbersome cocktail of reagents. In fact, according to the experimental procedure, the reaction is not so complicated. They mix the alkyl tosylate, NiBr2·glyme (commercial), 4,4?-di-tert-butyl-2,2?-bipyridine, KI and Mn powder (purchased and used directly), add DMA (from the bottle) and 4-ethylpyridine (they distill this out, yes), the (hetero)arylbromide and heat at 80 °C for 18 h. Sounds complicated, but they have good results, with yields ranging from 38 to 69% after purification. Most of the examples in their tables include heterocycles (indole, quinoline, benzothiophene, benzofurane, pyridine, etc.).
I envision a couple of compounds where we can use this protocol right away…
J. Org. Chem. 2015, 80 (5), pp 2907-2911.
See: 10.1021/acs.joc.5b00135