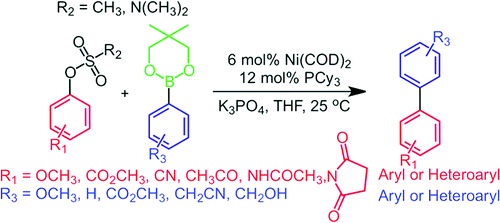
Ni(COD)2/PCy3 Catalyzed Cross-Coupling of Aryl and Heteroaryl Neopentylglycolboronates with Aryl and Heteroaryl Mesylates and Sulfamates in THF at Room Temperature
Coupling of arylmesylates and arylsulfamates using a Nickel catalyst.
If it was not clear seeing the previous entry in this top ten, let’s say it clearly: We can couple now almost anything!!!! This paper by Percec (University of Pennsylvania, Penn., USA) is another step forward. As in another example of our previous issue, the substrates here are also mesylates. Additionally, you can use sulfamates instead. The catalytic system is comprised of Ni(COD)2 and PCy3. The boron partners are not boronic acids (that has been published before), but neopentylglycolboronates. The reason you can find it in the introduction of the partner. To summarize, 23 examples with different mesylates are reported including, take note, hindered mesylates, substrates that are not easily coupled with the usual Suzuki conditions using Palladium. The reaction works nicely with those substrates and is performed in THF at 25 °C !! Another 23 examples with arylsulfamates are reported, also with excellent yields, and the icing on the cake are 18 examples of the coupling with different heterocycles (pyridines, quinolines, isoquinolines and thiophenes).
J. Org. Chem., Article ASAP. See: 10.1021/jo202037x