Iron Complex-Catalyzed N-Arylation of Pyrazoles under Aqueous Medium
Interesting protocol for the iron-catalyzed coupling of pyrazoles in aqueous medium.
In the last twenty years, there has been increasing interest in the development of Green Chemistry, that is, “The invention, design, and application of chemical products and processes to reduce or eliminate the use and generation of hazardous substances”. In other words, replacing toluene by water is green chemistry. Or changing palladium by iron.
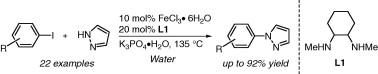
The paper by Kwong (Hong Kong, China) is a good example of green chemistry. The authors have developed a protocol to carry out the iron-catalyzed coupling of pyrazole with aryl iodides in aqueous medium, as a way to overcome the problems found with solventless conditions. In a typical reaction, aryl iodide and pyrazole are reacted with FeCl3·6H2O (10 mol%), trans–N,N‘-dimethyl-1,2-diaminocyclohexane (20 mol%), and K3PO4·H2O in water at 135 °C. The scope of the reaction seems to be good, allowing different groups in the aryl iodide. Some nitrogen-containing heteroaryl iodides are also used as substrates, and the amine partners can be not only pyrazole, but indole, azaindole, or carbazole, among others. Unfortunately, no additional data on the coupling of substituted pyrazoles, specially regarding regioselectivity, are provided.
Tetrahedron Lett. 50 (2009) 5868–5871. See: 10.1016/j.tetlet.2009.08.018