Direct Synthesis of Cbz-protected (2-Amino)-6-(2-aminoethyl)pyridines
Construction of ethylamino chains over functionalized pyridines and other azoles.
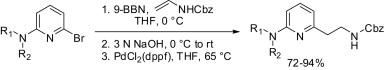
The importance of the 2-amino-6-(2-aminoethyl)pyridine skeleton is well known to medicinal chemists. This important structural motif is present among several medicinaly important molecules covering very different therapeutic areas. Many synthetic approaches can be found in the literature, but none of them is general and capable of render many different analogs directly. The work of Roy et al. (AMRI, NY, USA) is a very elegant and interesting method to solve this synthetic problem.
The work by Roy, done in collaboration with chemists from Schering-Plough (i.e., Merck) is centered around the introduction of the ethylamino chain into 2-bromopyridines bearing a 6-amino substituent; many substrates of this type are commercially available. After exploring some alternatives, they focused in a Suzuki-Miyaura coupling between the pyridine and a borane derived from benzyl N-vinylcarbamate. The final protocol involves reacting benzyl N-vinylcarbamate with 9-BBN at 0 °C, followed by treatment with aqueous NaOH. The mixture is then transferred to a solution of the 2-amino-6-bromopyridine and PdCl2(dppf) (10 mol%) in THF at r.t. and heated at 65 °C for 24 h. NaOH seems to be the key base for the reaction, since no coupling is observed with K2CO3. Final compounds are obtained in excellent yields and the reaction can be applied also to diazines: One example of a pyrazine and three examples with pyrimidines are shown.
Tetrahedron 2010 , 66 (11), Pages 1973-1979. See: 10.1016/j.tet.2010.01.025