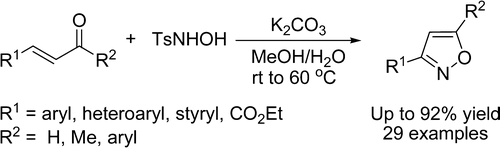Efficient and Regioselective One-Pot Synthesis of 3-Substituted and 3,5-Disubstituted Isoxazoles
A new one-pot procedure for the synthesis of isoxazoles with reverted regiochemistry.
The isoxazol ring is a common motif in many natural and man-made compounds with biological activity. I remember synthetizing one many years ago, during my lab classes as a chemistry student. The reaction involved a [3+2] cycloaddition of an alkene (in fact, an enamine) with a nitrile oxide. Years later, we have prepared other isoxazoles following the usual path, condensation of a,b-unsaturated carbonyl compounds with hydroxylamine. However, this method has some limitations regarding the type of compounds you can prepare.
She (Lanzhou, China) has published a new method deceptively simple for the one-pot preparation of 3- and 3,5-disubstituted isoxazoles. Deceptive regarding its potential, not the experimental simplicity, because the protocol is straightforward. It relies on using N-tosylhydroxylamine instead hydroxylamine and the consequences are striking. First, the regiochemistry is reversed, giving the opposite isomer that would be obtained with the common reaction. Second, it is necessary to have a substituent in the beta position of the unsaturated compound, which becomes position 3 in the isoxazol, and 3-isoxazoles are hard to prepare by other methods. And third, no regioisomers are formed. According to the authors, everything is due to the tosyl substituent.
Although the initial method had two steps, formation of an isoxazoline and oxidation of the ring, the protocol has been modified so mixing the carbonyl compound, excess of N-tosylhydroxylamine and K2CO3 in MeOH/H2O at 40–60 °C for 24 h, yields the final compounds. Twenty nine examples are reported with different substituents, including other heterocycles.
Org. Lett., 2009, 11 (17), pp 3982–3985. See: 10.1021/ol901626n