Palladium-Catalyzed Benzylation of Heterocyclic Aromatic Compounds
A method for the palladium-catalyzed benzylation of heterocycles.
During the years GalChimia has been carrying out literally hundreds of projects, some of them involving a transformation have always given me the same feeling of inefficiency: the introduction of a benzyl substituent on an aromatic ring. Often, the reaction used is the Friedel–Crafts, a classic reaction that I have used many times in the past with mixed results. Others, it was the palladium-catalyzed introduction of an enol ether, followed by hydrolisis of the enol ether. And then, always, the reduction of the carbonyl group.
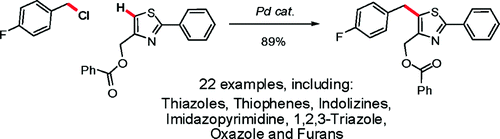
This method by Fagnou (Ottawa, Canada) has been specially developed for its use on heteroaromatic compounds, which usually do not stand against the harsh conditions of Friedel–Crafts reactions. It does not need a halogen also, so you can carry out a coupling. Moreover, you do not need to reduce a ketone because a benzylic halide is used. The protocol involves the reaction of a benzyl chloride, the heteroarene, Pd(OPiv)2 as the catalyst (2 mol%), 2-Ph2P-2′-(Me2N)biphenyl (4 mol%), PivOH (20 mol%), and Cs2CO3 in toluene at 110 °C for 16–20 h. More than 20 examples are included, mostly sulfur-containing heterocycles, but furanes, triazoles, oxazoles and others are described, with yields ranging from good to excellent.
Org. Lett., 2009, 11 (18), pp 4160–4163. See: 10.1021/ol901689q