Pd-Catalyzed Conversion of Aryl Chlorides, Triflates, and Nonaflates to Nitroaromatics
A method for the introduction of a nitro group by palladium catalysis.
Nitro aromatic compounds are really useful for a number of reasons. They are usually prepared by electrophilic aromatic substitution using HNO3 and a strong acid or with dinitrogen pentoxide. This is probably one of the oldest organic chemistry reactions but, as many chemists know, this method suffers from several issues, mainly harsh conditions and regioselectivity. And more important in the realm of medicinal chemistry, the electrophilic nitration does not work with many heteroaromatic compounds. For example, pyridines can not be nitrated directly, and the usual route for a nitropyridine involves preparation of the N-oxide, nitration and reduction of the oxide back to the pyridine. The development of a simpler, more effective nitration method is therefore important, and some authors have reported additional protocols but their scope is limited.
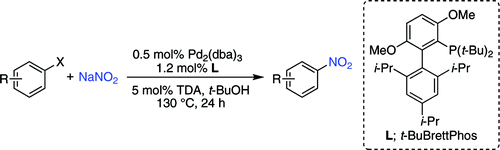
The group of Buchwald (Boston, USA) has published now a new protocol using palladium (what else?) for the conversion of aryl chlorides, triflates, and nonaflates into nitroaromatic compounds. A typical reaction involves mixing Pd2(dba)3, t-BuBrettPhos, and sodium nitrite under inert atmosphere. Then, the substrate, tris(3,6-dioxaheptyl)amine (TDA), which is a PTC critical for the solubility of the reaction, and t-BuOH are added to the flask. The reaction is heated at 130 °C for 24 h, giving the products in yields from very good to excellent. About 20 examples are described, included some heterocycles: quinoline, unprotected indoles, and carbazole. Interestingly, no examples on pyridines are reported… and no comment is made on the paper. Even thus, it is an interesting addition to the medicinal chemist weaponry.
J. Am. Chem. Soc., 2009, 131 (36), pp 12898–12899. See: 10.1021/ja905768k